June 2022 Provider News
Date: 06/30/22
In this issue:
- Billing Updates - Inactive NPIs: Requirement for Revalidation
- Quality - Well Child Visits/Immunizations, 90 Day Refills
- Apple Health Core Connections - What Primary Care Providers Need to Know for Youth Entering Foster Care, Reminder: DCYF COVID Vaccine Process for Youth in Foster Care
- Clinical Policy - Clinical Policy Announcements with Prior Authorization Implications, Clinical Policy Updates
- General Updates - National Imaging Associates Update, InterQual 2022, Enteral Formulas
- Training/Education - Centene’s Clinical Provider Training, Aligning Quality Measurement: Measuring What Matters, Apple Health After Pregnancy Coverage, Region 10 Opioid Summit
- Pharmacy Update - EA Codes for Contraceptives, Prior Authorization for Prescriptions Fax Number Change, and Flat Based Pricing Program
______________________________________________________________________________________
Billing Updates
Inactive NPIs: Requirement for Revalidation
Beginning January 1, 2022, Coordinated Care denies all claims submitted with an inactive NPI.
The Affordable Care Act (ACA) requires state Medicaid agencies to revalidate the enrollment of all Medicaid providers once every five years. To avoid any potential denials for an inactive NPI, providers must respond to HCA’s notices to complete revalidation. Upon receiving a revalidation notification, providers should gather and submit all documents requested on their Revalidation Checklist, complete the online revalidation process, and complete any other documents that may be required specific to their provider type. More information on the revalidation process can be found here.
If providers did not respond to their revalidation notification and therefore became inactive in ProviderOne, Coordinated Care began denying their claims on January 1, 2022. Please note that on Professional HCFA Claims, both the Rendering (box 24j) & Billing (box 33a) NPIs need to be active for payment. For Facility UB Claims, both Billing (box 56) & Attending (box 76) NPIs need to be active for payment. If either the individual or group NPI is inactive, the entire claim is denied EXnZ (denial will show as: NPI NOT REGISTERED. COMPLETE NPI ENROLLMENT/REVALIDATION PROCESS W/ HCA).
If a provider’s enrollment with HCA lapsed or they never enrolled with HCA, providers should complete the enrollment process specific to their provider type. More information about enrolling as a Medicaid Provider can be found on HCA’s site.
Please note: HCA will review requests for retro-active registration of providers if a provider’s registration has lapsed. A provider needs to complete the Effective Date Change form and other required documentation to be considered for retro-active registration.
As of March 2022: HCA is experiencing an extraordinarily high volume of applications and there is no automated way to track the status of pending applications. Coordinated Care and the HCA ask all providers to confirm on the application, the provider’s desired effective date they need their enrollment to start. Please do not call HCA to confirm receipt of your application, unless you did not receive the following message when you submitted your application:
Claims denied EXnZ will be reprocessed for providers whose retro-active registration is approved. This eliminates the need for providers to resubmit their claims or request reprocessing.
Please reach out to your Provider Network Specialist if you have any questions about the revalidation process.
______________________________________________________________________________________
Quality
Well Child Visits and Immunizations
Coordinated Care is ready to help your clinic identify patients with open care gaps in the following categories:
- Well Child Visits
- Immunizations
Please contact our EPSDT Coordinator, Eboney, for details: Eboney.A.ReeceHerrera@coordinatedcarehealth.com.
Asthma and 90 Day Medication Refills
Moving patients with asthma to 90-day refills may help increase their adherence to maintenance medication.
______________________________________________________________________________________
Apple Health Core Connections
Coordinated Care is the only managed care organization administering the Integrated Apple Health Foster Care program in collaboration with the Health Care Authority and Department of Children Youth and Families. Our program, Apple Health Core Connections, serves children and youth in foster care, adoption support, alumni of foster care (ages 18-26), and children reunified with their parents and youth in the Unaccompanied Refugee Minor program. Providers and members can reach us at 1-844-354-9876 or AHCCTeam@coordinatedcarehealth.com.
The Coordinated Care Community Education (CE) Team serves providers and their staff with training to support the needs of the Medicaid population. The CE Team can offer training on topics such as Adoption Success, Trauma Informed Care, ACEs, Resilience, and Secondary Trauma and Self Care. To request more information or to schedule no-cost training please email communityeducation@coordinatedcarehealth.com.
What Primary Care Providers Need to Know for Youth Entering Foster Care
Youth entering foster care have unique needs, and we want to support providers seeing the youth at one of the most traumatic moments of their lives. The Well-Child/EPSDT exam is the main way that primary care providers can help youth in a trauma-minimizing way.
Requirements for Foster Care EPSDT within 30 days
Here are some key points about EPSDT exams for youth entering out-of-home (foster) care:
- Youth must receive an EPSDT exam ASAP upon placement into foster care
- Whenever possible, if a Well-Child Exam (EPSDT) can occur within the first five days of entering care, this is a huge help to the youth, caregivers, and Department of Children, Youth, and Families (DCYF) Caseworkers. - Appointment and documentation must be completed within 30 days
- Bill as EPSDT exam, not as establishing care or office visit
- There is no benefit maximum on EPSDT exam. Coordinated Care will pay for EPSDT exams as often as necessary for youth in foster care.
- An EPSDT exam may be needed after a child changes placement - Payment for the EPSDT will be made even if the provider is not the assigned PCP
EPSDT and CHET
- Child Health Education and Tracking (CHET) Screeners are specialized caseworkers in DCYF authorized to collect health information for the CHET report.
- Due 30 days from the day a youth enters foster care, the CHET report must include the record from the EPSDT well-child exam.
- Exam must be billed only as EPSDT - CHET Screeners will send fax or email request noting WAC 182-502-0020 –Health Care Record Requirements, authorizing them to receive records.
- It’s critical to send as soon as possible; consider an office or clinic system process to ensure copies are sent promptly for youth in foster care. - A complete EPSDT record that fulfills the state requirement must include:
- Vitals
- Review of symptoms
- Include any abnormal findings
- Recommendations/Referrals
- Including anticipatory guidance
- Make sure all referrals are in Coordinated Care's network - Please note that most Tribes do not use the CHET process
EPSDT Exams for Foster Care
We encourage all providers and billing teams to use the TJ modifier for any youth in foster care to get the enhanced rate.
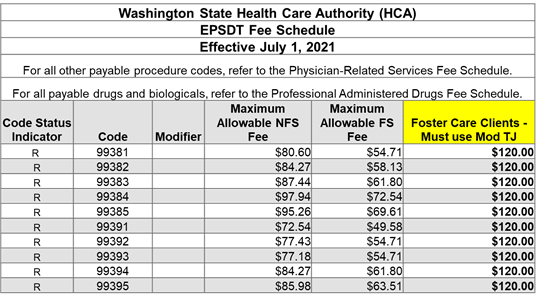
https://www.hca.wa.gov/assets/billers-and-providers/epsdt-20210701.xlsx
If your team has any questions about working with Apple Health Core Connections members, please reach out to your Provider Relations Representative. We’re happy to provide support and training.
Reminder: DCYF COVID Vaccine Process for Youth in Foster Care
As we anticipate the FDA and CDC emergency authorization of the Pfizer and Moderna COVID vaccines for youth under 5 years of age, we want to remind providers of the process for vaccinating youth in out-of-home/foster care.
The Washington State Department of Children, Youth, and Families (DCYF) has guidance for youth in out-of-home (foster) care to get consent for and access to COVID vaccinations. (Youth ages 18 and up do not need any additional consent to receive the vaccine.)
Primary care providers and vaccination sites may be asked to vaccinate youth in foster care, and youth will arrive with caregivers with the Youth and Parent Consent forms shown below. Youth under age 13 do not need to have the Youth Consent Form, only the Parent Consent form or a court order for the vaccine. Examples of the forms are below.
If a bio-parent does not consent for a youth to receive the vaccine, or if a court order is needed, you may also be asked to determine if a youth is healthy enough to receive the COVID vaccine. This consultation can occur in an office visit or by telehealth. DCYF will accept your COVID Vaccine recommendation on a Visit Summary as documentation.
For more information, please contact:
- Jen Estroff, AHCC Liaison: jestroff@coordinatedcarehealth.com, 206-492-9019
- Apple Health Core Connections Care Coordination Team: AHCCTeam@coordinatedcarehealth.com
DCYF - COVID Vaccine Guidance for Children in Care


______________________________________________________________________________________
Clinical Policy
Clinical Policy Announcements with Prior Authorization Implications:
The below notice has been updated to reflect a new effective date of October 1, 2022. Previous announcements reflected a September 1, 2022, effective date.
- Interventional Pain Management
- Left Heart Catheterization
- Cardiac Implants
Effective October 1, 2022, interventional pain management, left heart catheterization and cardiac implant services will be reviewed by National Imaging Associates, Inc. (NIA) to determine if the services are medically necessary and a covered service under Coordinated Care health plans. You will find the policies on the NIA website. A link is available under “provider resources” on CoordinatedCareHealth.com. Policy names are listed below under Monthly Updates.
CPT codes considered interventional pain management for purposes of this review are: 0213T-0218T, 0228T-0231T, G0260, 27096, 62320-62323, 64479, 64480, 64483, 64484, 64490-64495, 64633-64636. All codes currently require prior authorization, but effective 10/1/22, authorization will be provided by NIA.
CPT codes considered left heart catheterization for purposes of this review are: 93452-93459, 93460-93464, 93565-933568. Prior authorization, issued by NIA, will be required effective 10/1/22.
CPT codes considered cardiac implants for purposes of this review are: 33221, 33224, 33225, 33231, 33230, 33240, 33249, 33206-33208, 33212, 33213. Prior authorization, issued by NIA, will be required effective 10/1/22.
Reminders
Genetic Testing: Twenty-seven new genetic testing policies will be implemented on July 1, 2022, and are listed below. The policies can be found on our provider website. Previously posted genetic testing policies will be archived as of June 30.
Cell-free Fetal DNA: The Health Care Authority does not fund testing for all pregnancies. Consequently, Coordinated Care requires prior authorization for cell-free fetal DNA testing for our Apple Health members. The current policy (WA.CP.MP.84) will be archived 6/30/22 and replaced with WA.CP.MP.231 – Genetic Testing Non-Invasive Prenatal Screening (NIPS) as part of the genetic testing policy update, but the prior authorization requirements will not change.
Clinical Policy Monthly Updates
The below clinical policies received updates as part of our regular monthly review in June. These policy changes are effective July 1, 2022. You will find the policies, including a description of the revisions, posted on the policy site.
| Policy Number | Policy Title | Line of Business |
WA.CP.MP.509 | Upper GI Endoscopy for GERD | Apple Health |
WA.CP.MP.518 | Negative Pressure Wound Therapy for Home Use | Apple Health |
CP.MP.110 | Bronchial Thermoplasty | Ambetter |
CP.MP.116 | Lysis of Epidural Lesions | Apple Health & Ambetter |
CP.MP.132 | Heart-Lung Transplant | Apple Health & Ambetter |
CP.MP.210 | Repair of Nasal Valve Compromise | Apple Health & Ambetter |
CP.MP.58 | Intestinal and Multivisceral Transplant | Apple Health & Ambetter |
CP.MP.147 | Percutaneous Left Atrial Appendage Closure Device for Stroke Prevention | Apple Health & Ambetter |
CP.MP.176 | Outpatient Cardiac Rehabilitation | Ambetter |
CP.MP.188 | Pediatric Oral Function Therapy | Apple Health & Ambetter |
CP.MP.100 | Allergy Testing and Therapy | Apple Health & Ambetter |
The below new policy was approved as part of our regular monthly review in June. This policy will be effective October 1, 2022. You will find the policy posted on the policy site.
| Policy Number | Policy Title | Line of Business |
CP.MP.244 | Liposuction of Lipedema | Apple Health & Ambetter |
TThe below new policy was approved as part of our regular monthly review in June. This policy will be effective July 1, 2022. You will find the policy posted on the policy site.
| Policy Number | Policy Title | Line of Business |
WA.CP.MP.38 | Ultrasound in Pregnancy | Ambetter |
The below policy will be archived effective July 1, 2022. It is being replaced with WA.CP.MP.38.
| Policy Number | Policy Title | Line of Business |
CP.MP.38 | Ultrasound in Pregnancy | Ambetter |
The below policies were previously announced as revised on the dates noted. You will find the policies posted on the policy site.
| Policy Number | Policy Title | Effective Date | Line of Business |
CP.MP.86 | Neonatal Abstinence Syndrome Guidelines | 7/1/22 | Apple Health & Ambetter |
| CP.MP.243 | Implantable Loop Recorder (Implantable Cardiac Monitor) | 9/1/22 | Apple Health & Ambetter |
New Genetic Testing Policies effective 7/1/22:
| Policy Number | Policy Title | Line of Business |
CP.MP.215 | Genetic Testing Aortopathies & Connective Tissue Disorder | Apple Health & Ambetter |
CP.MP.216 | Genetic Testing Cardiac Disorder | Apple Health & Ambetter |
CP.MP.217 | Genetic Testing Dermatologic Conditions | Apple Health & Ambetter |
CP.MP.218 | Genetic Testing Epilepsy, Neurodegenerative and Neuromuscular Disorders | Apple Health & Ambetter |
WA.CP.MP.219 | Genetic Testing Exome and Genome Sequencing (addresses previous policy WA.CP.MP.524 - Whole Exome Sequencing) | Apple Health |
CP.MP.219 | Genetic Testing Exome and Genome Sequencing | Ambetter |
CP.MP.220 | Genetic Testing Eye Disorders | Apple Health & Ambetter |
CP.MP.221 | Genetic Testing Gastroenterologic Disorders (non-cancerous) | Apple Health & Ambetter |
CP.MP.222 | Genetic Testing General Approach to Genetic Testing | Apple Health & Ambetter |
CP.MP.223 | Genetic Testing Hearing Loss | Apple Health & Ambetter |
CP.MP.224 | Genetic Testing Hematologic Conditions (non-cancerous) | Apple Health & Ambetter |
CP.MP.225 | Genetic Testing Hereditary Cancer Susceptibility | Apple Health & Ambetter |
CP.MP.226 | Genetic Testing Immune, Autoimmune and Rheumatoid Disorders | Apple Health & Ambetter |
CP.MP.227 | Genetic Testing Kidney Disorders | Apple Health & Ambetter |
CP.MP.228 | Genetic Testing Lung Disorders | Apple Health & Ambetter |
CP.MP.229 | Genetic Testing Metabolic, Endocrine and Mitochondrial Disorders | Apple Health & Ambetter |
| CP.MP.230 | Genetic Testing Multisystem Inherited Disorders, Intellectual Disability and Developmental Delay | Ambetter |
| WA.CP.MP.230 | Genetic Testing Multisystem Inherited Disorders, Intellectual Disability and Developmental Delay (addresses previous WA.CP.MP.512- Genomic Microarray Testing) | Apple Health |
| WA.CP.MP.231 | Genetic Testing Non-Invasive Prenatal Screening (NIPS) (addresses previous policy WA.CP.MP.84 – Cell-free Fetal DNA Testing) | Apple Health |
| CP.MP.231 | Genetic Testing Non-Invasive Prenatal Screening (NIPS) | Ambetter |
| CP.MP.232 | Genetic Testing Pharmacogenetics | Apple Health & Ambetter |
| CP.MP.233 | Genetic Testing Pre-Implantation | Apple Health & Ambetter |
| CP.MP.234 | Genetic Testing Prenatal and Preconception Carrier Screening | Apple Health & Ambetter |
| CP.MP.235 | Genetic Testing Prenatal Diagnosis (via Amniocenteses, CVS or PUBS) and Pregnancy Loss | Apple Health & Ambetter |
| CP.MP.236 | Genetic Testing Skeletal Dysplasia and Rare Bone Disorders | Apple Health & Ambetter |
| CP.MP.237 | Oncology Algorithmic Testing (addresses previous policy WA.CP.MP.511 - Gene Expression Profile Testing for Cancer Tissue) | Apple Health & Ambetter |
| CP.MP.238 | Oncology Cancer Screening | Apple Health & Ambetter |
| CP.MP.239 | Oncology Circulating Tumor DNA and Circulating Tumor Cells (Liquid Biopsy) | Apple Health & Ambetter |
| CP.MP.240 | Oncology Cytogenetic Testing | Apple Health & Ambetter |
| CP.MP.241 | Oncology Molecular Analysis of Solid Tumors and Hematologic Malignancies | Apple Health & Ambetter |
Coordinated Care Genetic Testing Policies to be archived effective 6/30/22:
| Policy Number | Policy Title | Line of Business |
WA.CP.MP.84 | Cell-free Fetal DNA Testing | Apple Health |
CP.MP.84 | Cell-free Fetal DNA Testing | Ambetter |
CP.MP.89 | Genetic and Pharmacogenetic Testing | Apple Health & Ambetter |
| WA.CP.MP.511 | Gene Expression Profile Testing for Cancer Tissue | Apple Health |
| WA.CP.MP.512 | Genomic Microarray Testing | Apple Health |
WA.CP.MP.524 | Whole Exome Sequencing | Apple Health |
New NIA Pain Management and Cardiac policies to be effective 10/1/22:
| Policy Number | Policy Title | Line of Business |
NIA_CG_300 | Epidural Spine Injections | Apple Health & Ambetter |
NIA_CG_301 | Paravertebral Facet Point Injections or Blocks | Apple Health & Ambetter |
NIA_CG_302 | Paravertebral Facet Joint Denervation (Radiofrequency Neurolysis) | Apple Health & Ambetter |
| NIA_CG_303 | Sacroiliac Joint Injections | Apple Health & Ambetter |
| NIA_CG_065 | Heart Catheterization | Apple Health & Ambetter |
NIA_CG_322 | Pacemaker | Apple Health & Ambetter |
| NIA_CG_321 | Implantable Cardioverter Defibrillator (ICD) | Apple Health & Ambetter |
| NIA_CG_320 | Cardiac Resynchronization Therapy | Apple Health & Ambetter |
Coordinated Care Pain Management policies to be archived 9/30/22:
| Policy Number | Policy Title | Line of Busines |
CP.MP.164 | Caudal or Interlaminar Epidural Steroid Injections for Pain Management | Apple Health & Ambetter |
CP.MP.165 | Selective Nerve Root Blocks and Transforaminal Epidural Injections for Pain Management | Apple Health & Ambetter |
WA.CP.MP.171 | Facet Joint Interventions for Pain Management | Apple Health |
| CP.MP.171 | Facet Joint Interventions for Pain Management | Ambetter |
| CP.MP.166 | Sacroiliac Joint Interventions for Pain Management | Apple Health & Ambetter |
______________________________________________________________________________________
General Updates
National Imaging Associates Update
National Imaging Associates, Inc. (NIA) provides utilization management services for non-emergent, advanced diagnostic imaging services for Coordinated Care and Ambetter. In the interest of streamlining authorization processes and improving member outcomes, Coordinated Care has expanded its partnership with NIA. In addition to the procedures that currently require prior authorization through NIA, interventional pain management, left heart catheterizations, and implantables will also require prior authorization beginning October 1, 2022.
Program Components
- Evidence-based clinical guidelines and proprietary algorithms to support clinically appropriate options for each member.
- Clinical reviews will be conducted by NIA board-certified internists with specialized cardiac training and board-certified cardiologists related to elective cardiac diagnostic imaging when peer-to-peer review is required.
Prior authorization through NIA is currently required for these outpatient advanced diagnostic imaging procedures:
- CT/CTA
- MRI/MRA
- PET Scan
- Myocardial Perfusion Imaging (MPI)
- CCTA
Effective October 1, 2022, the following services will require prior authorization through NIA:
- Interventional Pain Management
- Left Heart Catheterization
- Cardiac Implantable Devices (defibrillator, pacemaker)
We are confident this program will have a positive impact on the quality of care rendered to your members, and we look forward to working with you to deliver positive outcomes to our community. We would be happy to discuss the program further. Should you have questions, please contact Coordinated Care at 1-877-644-4643.
Utilization Management
All UM Reviews using Acute/Post-Acute and Ambulatory Care Planning criteria for reviews moved from Change Healthcare InterQual 2021 to InterQual 2022 on June 15, 2022. Please contact Leona Parker, UM Director (Leona.M.Parker@CoordinatedCareHealth.com) with any questions.
Utilization Management Pre-Service Updates
ALERT TO ALL ENTERAL PROVIDERS: In accordance with the changes to WAC 182-554-400 Enteral Nutrition, beginning August 27, 2022, Coordinated Care will allow up to a 10-day overlap in dates for processing of claims for refills delivered/shipped prior to the member exhausting their supply.
The Provider Operations Manual for Washington Apple Health (Medicaid) and Foster Care has been updated and republished as the current 2022 edition. It is effective on July 1, 2022.
Coordinated Care Medicaid and Foster Care
______________________________________________________________________________________
Training/Education
Visit Centene’s Clinical Provider Training website to find webinars available to all Coordinated Care providers. Webinars are on clinical behavioral health topics and usually offer free continuing education hours. Click on the “National Provider Webinars” button on the right side of the page and scroll to find a complete listing.
Foundation for Health Care Quality presents Aligning Quality Measurement: Measuring What Matters. We will start with a national perspective with Tamyra Garcia, MPH, Deputy Director, Quality Measurement and Value-Based Incentives, Centers for Medicare and Medicaid Services and end with a boots on the ground panel to explore and discuss how aligned quality measurement can and should improve care delivery with Dr. Edwin Carmack, Confluence Health. July 21, 2022 10-11:30am. Register here.
WA State HCA recently hosted a webinar on Apple Health After Pregnancy Coverage. Watch the 16 minute recording.
Region 10 Opioid Summit. The virtual summit is an opportunity for professionals from Alaska, Idaho, Oregon, Washington, Indian Nations, urban Indian health programs, and recognized American Indian organizations to meet and explore ways to address the opioid crisis. August 3-4, 2022. Register here.
______________________________________________________________________________________
Pharmacy Update
EA Codes for Contraceptives
As of 6/1/2022, an expedited authorization (EA) code is required on all claims for contraceptives. The EA code will allow pharmacies to submit a claim for contraceptives based on the following criteria.
| Product | EA Code | Code Criteria |
Contraceptives | 85000000131 | Used as a contraceptive, dispense 1 year
|
| Contraceptives | 85000000132 | Used as a contraceptive, dispensed less than a twelve month supply due to ONE of the following:
|
| Contraceptives | 85000000133 | Used for other diagnosis, not related to contraception up to a 91-day supply. |
Prior Authorization for Prescriptions Only Fax Number Change Notice
The prior authorization for prescriptions ONLY fax number for Coordinated Care will be changing effective 07/05/022. The new fax number will be 1-833-645-2734.
Flat Based Pricing Program
Effective 8/01/2022, Coordinated Care will be implementing a Flat Based Pricing Program to reduce health care costs while maintaining member access to vital medication and managing patient care.
Drug pricing for different strengths of the same medication can vary, but there are times where a flat-price is applied across all strengths. The Flat Based Pricing Program is intended to minimize medication waste for identified flat-priced drugs where members are receiving an increased quantity of a lower-strength, when a smaller quantity can be dispensed from a high strength.
When a prescription for a flat-priced drugs is processed, the claim rejects and the pharmacy is instructed to adjust the drug strength accordingly. To resolve the claim rejection, the pharmacy will process the claim using a high strength; ex. Initial claim for 2 tablets of 15mg daily will need to be resubmitted for 1 tablet of 30mg daily.
The following drugs will have a quantity limit update as part of the Flat Based Pricing Program effective 8/01/2022.
Product Name | Generic Product Identifier Name | Quantity Limit Effective 08/01/2022 |
AUBAGIO | Teriflunomide Tab 7 MG | 1 tablet/day |
AUBAGIO | Teriflunomide Tab 14 MG | 1 tablet/day |
BAFIERTAM | Monomethyl Fumarate Capsule Delayed Release 95 MG | 4 capsules/day |
CABOMETYX | Cabozantinib S-Malate Tab 20 MG (Base Equivalent) | 1 tablet/day |
CABOMETYX | Cabozantinib S-Malate Tab 60 MG (Base Equivalent) | 1 tablet/day |
ELIQUIS | Apixaban Tab 2.5 MG | 2 tablets/day |
ELIQUIS | Apixaban Tab 5 MG | 2 tablets/day |
FARXIGA | Dapagliflozin Propanediol Tab 5 MG (Base Equivalent) | 1 tablet/day |
FARXIGA | Dapagliflozin Propanediol Tab 10 MG (Base Equivalent) | 1 tablet/day |
GILENYA | Fingolimod HCl Cap 0.25 MG (Base Equiv) | 1 capsule/day |
GILENYA | Fingolimod HCl Cap 0.5 MG (Base Equiv) | 1 capsule/day |
ICLUSIG | Ponatinib HCl Tab 10 MG (Base Equiv) | 1 tablet/day |
ICLUSIG | Ponatinib HCl Tab 15 MG (Base Equiv) | 1 tablet/day |
ICLUSIG | Ponatinib HCl Tab 30 MG (Base Equiv) | 1 tablet/day |
ICLUSIG | Ponatinib HCl Tab 45 MG (Base Equiv) | 1 tablet/day |
IMBRUVICA | Ibrutinib Tab 140 MG | 1 tablet/day |
IMBRUVICA | Ibrutinib Tab 280 MG | 1 tablet/day |
IMBRUVICA | Ibrutinib Tab 420 MG | 1 tablet/day |
IMBRUVICA | Ibrutinib Tab 560 MG | 1 tablet/day |
INGREZZA | Valbenazine Tosylate Cap 40 MG (Base Equiv) | 1 capsule/day |
INGREZZA | Valbenazine Tosylate Cap 60 MG (Base Equiv) | 1 capsule/day |
INGREZZA | Valbenazine Tosylate Cap 80 MG (Base Equiv) | 1 capsule/day |
JAKAFI | Ruxolitinib Phosphate Tab 5 MG (Base Equivalent) | 2 tablets/day |
JAKAFI | Ruxolitinib Phosphate Tab 10 MG (Base Equivalent) | 2 tablets/day |
JAKAFI | Ruxolitinib Phosphate Tab 15 MG (Base Equivalent) | 2 tablets/day |
JAKAFI | Ruxolitinib Phosphate Tab 20 MG (Base Equivalent) | 2 tablets/day |
JAKAFI | Ruxolitinib Phosphate Tab 25 MG (Base Equivalent) | 2 tablets/day |
JANUMET | Sitagliptin-Metformin HCl Tab 50-500 MG | 2 tablets/day |
JANUMET | Sitagliptin-Metformin HCl Tab 50-1000 MG | 2 tablets/day |
JANUMET XR | Sitagliptin-Metformin HCl Tab ER 24HR 50-500 MG | 2 tablets/day |
JANUMET XR | Sitagliptin-Metformin HCl Tab ER 24HR 50-1000 MG | 2 tablets/day |
JANUMET XR | Sitagliptin-Metformin HCl Tab ER 24HR 100-1000 MG | 1 tablet/day |
JANUVIA | Sitagliptin Phosphate Tab 25 MG (Base Equiv) | 1 tablet/day |
JANUVIA | Sitagliptin Phosphate Tab 100 MG (Base Equiv) | 1 tablet/day |
JENTADUETO XR | Linagliptin-Metformin HCl Tab ER 24HR 2.5-1000 MG | 2 tablets/day |
LACOSAMIDE | Lacosamide Tab 150 MG | 2 tablets/day |
LACOSAMIDE | Lacosamide Tab 200 MG | 2 tablets/day |
LENVIMA 12MG DAILY DOSE | Lenvatinib Cap Therapy Pack 3 x 4 MG (12 MG Daily Dose) | 3 capsules/day |
LENVIMA 24 MG DAILY DOSE | Lenvatinib Cap Ther Pack 2 x 10 MG & 4 MG (24 MG Daily Dose) | 3 capsules/day |
LINZESS | Linaclotide Cap 72 MCG | 1 capsule/day |
LINZESS | Linaclotide Cap 145 MCG | 1 capsule/day |
LINZESS | Linaclotide Cap 290 MCG | 1 capsule/day |
LYNPARZA | Olaparib Tab 100 MG | 4 tablets/day |
LYNPARZA | Olaparib Tab 150 MG | 4 tablets/day |
MAYZENT | Siponimod Fumarate Tab 0.25 MG (Base Equiv) | 4 tablets/day |
OCALIVA | Obeticholic Acid Tab 5 MG | 1 tablet/day |
OCALIVA | Obeticholic Acid Tab 10 MG | 1 tablet/day |
ONGLYZA | Saxagliptin HCl Tab 2.5 MG (Base Equiv) | 1 tablet/day |
PRADAXA | Dabigatran Etexilate Mesylate Cap 75 MG (Etexilate Base Eq) | 2 capsules/day |
PRADAXA | Dabigatran Etexilate Mesylate Cap 150 MG (Etexilate Base Eq) | 2 capsules/day |
SAVAYSA | Edoxaban Tosylate Tab 15 MG (Base Equivalent) | 1 tablet/day |
SAVAYSA | Edoxaban Tosylate Tab 30 MG (Base Equivalent) | 1 tablet/day |
SAVAYSA | Edoxaban Tosylate Tab 60 MG (Base Equivalent) | 1 tablet/day |
VERZENIO | Abemaciclib Tab 50 MG | 2 tablets/day |
VERZENIO | Abemaciclib Tab 100 MG | 2 tablets/day |
VERZENIO | Abemaciclib Tab 150 MG | 2 tablets/day |
VERZENIO | Abemaciclib Tab 200 MG | 2 tablets/day |
VIMPAT | Lacosamide Tab 150 MG | 2 tablets/day |
VIMPAT | Lacosamide Tab 200 MG | 2 tablets/day |
VUMERITY | Diroximel Fumarate Capsule Delayed Release 231 MG | 4 capsules/day |
XARELTO | Rivaroxaban Tab 2.5 MG | 2 tablets/day |
XARELTO | Rivaroxaban Tab 10 MG | 1 tablet/day |
ZEPOSIA | Ozanimod HCl Cap 0.92 MG | 1 capsule/day |

